Threshold of antibodies considered good enough for protection?
0 comments
https://www.medrxiv.org/content/10.1101/2021.08.09.21261290v1.full.pdf
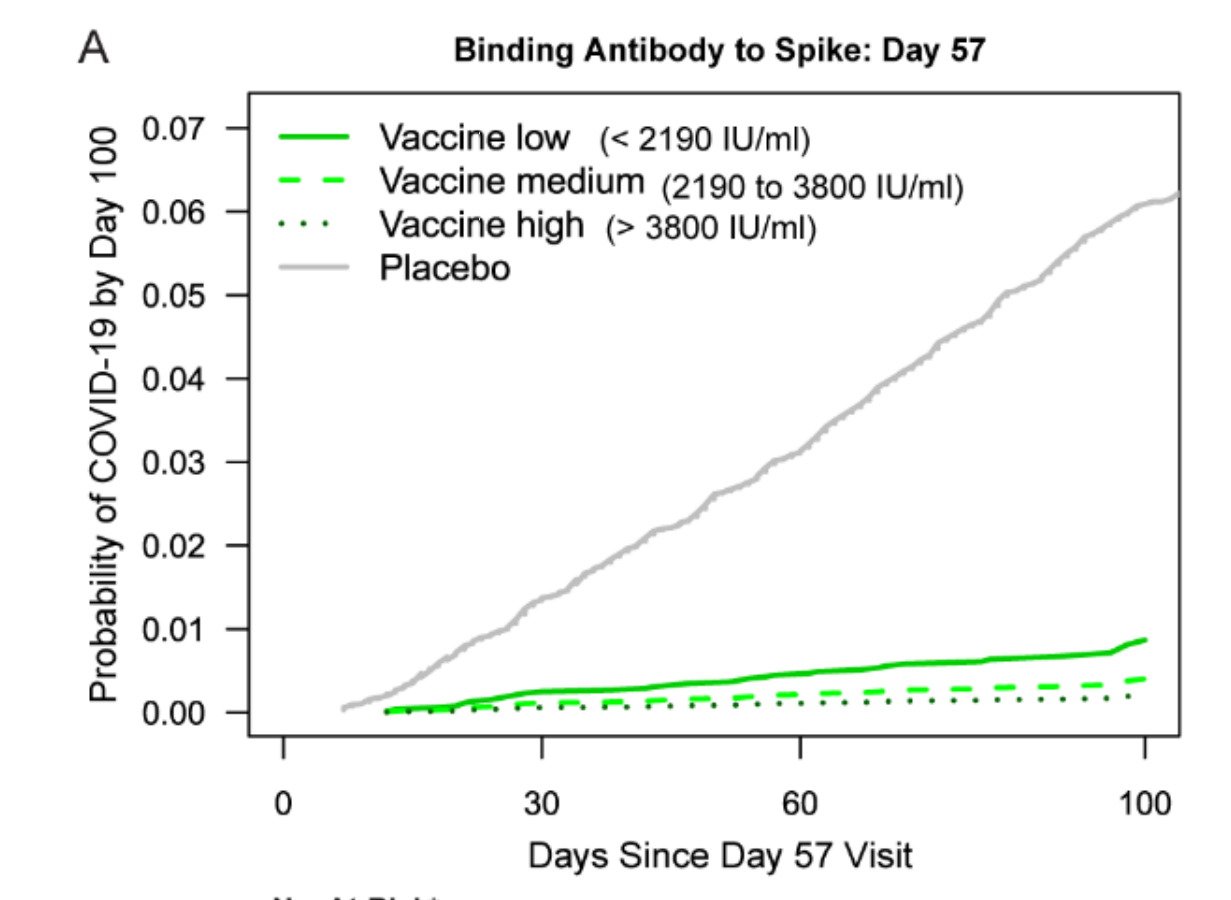
This study by Moderna correlates antibody levels in their COVE trial participants on day 57 (counting from the first dose) with vaccine efficacy from day 57 to day 100. This study is similar to a study previously done by AstraZeneca. Because the Moderna vaccine gives much higher antibody levels than does the AstraZeneca vaccine, the Moderna study gives us a lot more data at the higher end. It's also a good idea to do the same test for a different vaccine that works in a different way.
The reason these studies are being done is that there is a hope that antibodies in general or neutralizing antibodies will be a good enough predictor of vaccine efficacy that in the future we will be able to test new vaccines by giving a modest number of people the vaccine and then testing their antibody levels to make sure that they are in the range expected to be protective at a certain level. This would be much faster and cheaper than setting up a placebo-controlled trial with tens of thousands of people and waiting to see who gets covid.
These studies will also be helpful in eventually setting an official threshold of antibodies that are considered good enough to be protective. The WHO definitely has their eye on this goal, and has set international standards of antibody levels to facilitate comparison of antibody levels across countries.
Moderna found that when they correlated vaccine efficacy with neutralizing antibody levels calibrated to the WHO international standards, their result was very similar to AstraZeneca's result. That is, if you have a population of people who got AZ and another that got Moderna and the two populations are matched to have the same neutralizing antibody levels, the vaccine efficacy would be about the same for the two groups, even though the two vaccines are different in design and overall efficacy. This is good news for those who are hoping to test other vaccines without huge trials. The bad news for those people is that these trials were done long before the delta variant came along, and so they are only telling us how well a certain level of antibodies protects against older variants.
Moderna separated the trial participants into those with low, medium, and high levels of antibodies, and they give antibody levels in WHO international units. I am converting the international units into the Roche/labcorp units for ease of comparison with your own test if you've done one.
Remember, these are antibody levels at day 57, so they are higher than what you are used to seeing if you are testing months later than that. The vaccine efficacies given here are from day 57 to day 100. Overall vaccine efficacy for this time frame is 92.3%.
IgG antibodies to whole spike -- vaccine efficacy for group
Low: <1685 -- 88.3%
Medium: 1685 to 2923 -- 93.8%
High: >2923 -- 96.4%
IgG antibodies to receptor-binding domain (RBD) of spike -- vaccine efficacy for group
Low: <2546 -- 88.5%
Medium: 2546 to 4423 -- 94.3%
High: >4423 -- 96.0%
For all of the antibody measures, those with levels in the top 10% were about 10 times less likely to get covid as compared to the poorest responders (with antibody levels equivalent to 7.7 or lower on the Roche scale).
Those poorest antibody responders were still about half as likely to get covid compared to the placebo group. This is similar to what was seen with the AZ vaccine, where an antibody level of 10.8 (on the Roche scale) correlated to 50% efficacy.
The reason for this may be that something other than antibodies is helping. T-cells can't prevent infection, but they can stop an infection quickly enough that it isn't symptomatic, and this study is measuring efficacy against symptomatic covid. Another explanation may be because most people who got (pre-delta-variant) covid were only shedding small amounts of virus, so small amounts of antibodies might be fine to deal with the average contagious person you might encounter. Much higher antibody levels would be needed to deal with super-spreaders, which were rare with older variants. Unfortunately, although antibodies from the vaccine bind to the delta variant spike protein pretty well, the delta variant causes the average person to shed much larger amounts of virus, which appears to require higher levels of antibodies to prevent infection.
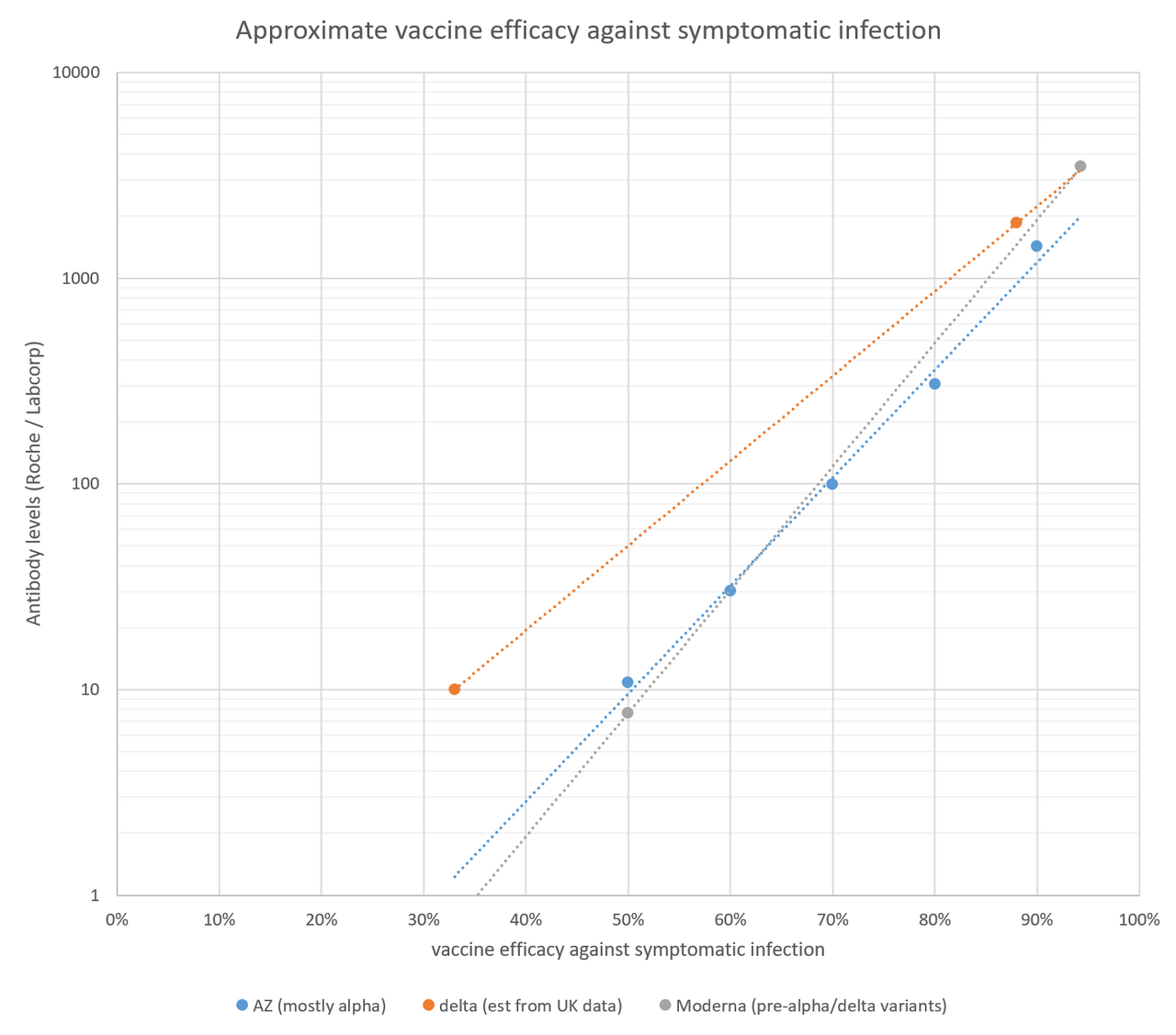
I added the Moderna results (for the RBD antibody test, since that is closest to the Roche test) to my handy graph to estimate vaccine efficacy from Roche/labcorp antibody levels. It doesn't really change anything, since the Moderna results are very similar to the AZ results, but it's nice to have some better data at the higher end of antibody levels. The data for the delta variant is based on the finding in the UK that the delta variant shifts vaccine efficacy for a single dose of Pfizer or AZ from 50% to 33%, and efficacy for two doses of Pfizer from 93% to 88%. If you want to see where your level correlates to this line, you can just find your antibody number on the Y axis (which is a log scale), see where it hits the orange line, and find the vaccine efficacy for that number on the X-axis. This line isn't perfect as it's based on just two data points, but it's the best that we have right now.
Comments